How Thunai AI Accelerates Regulatory Approvals With On-Prem Knowledge Engine
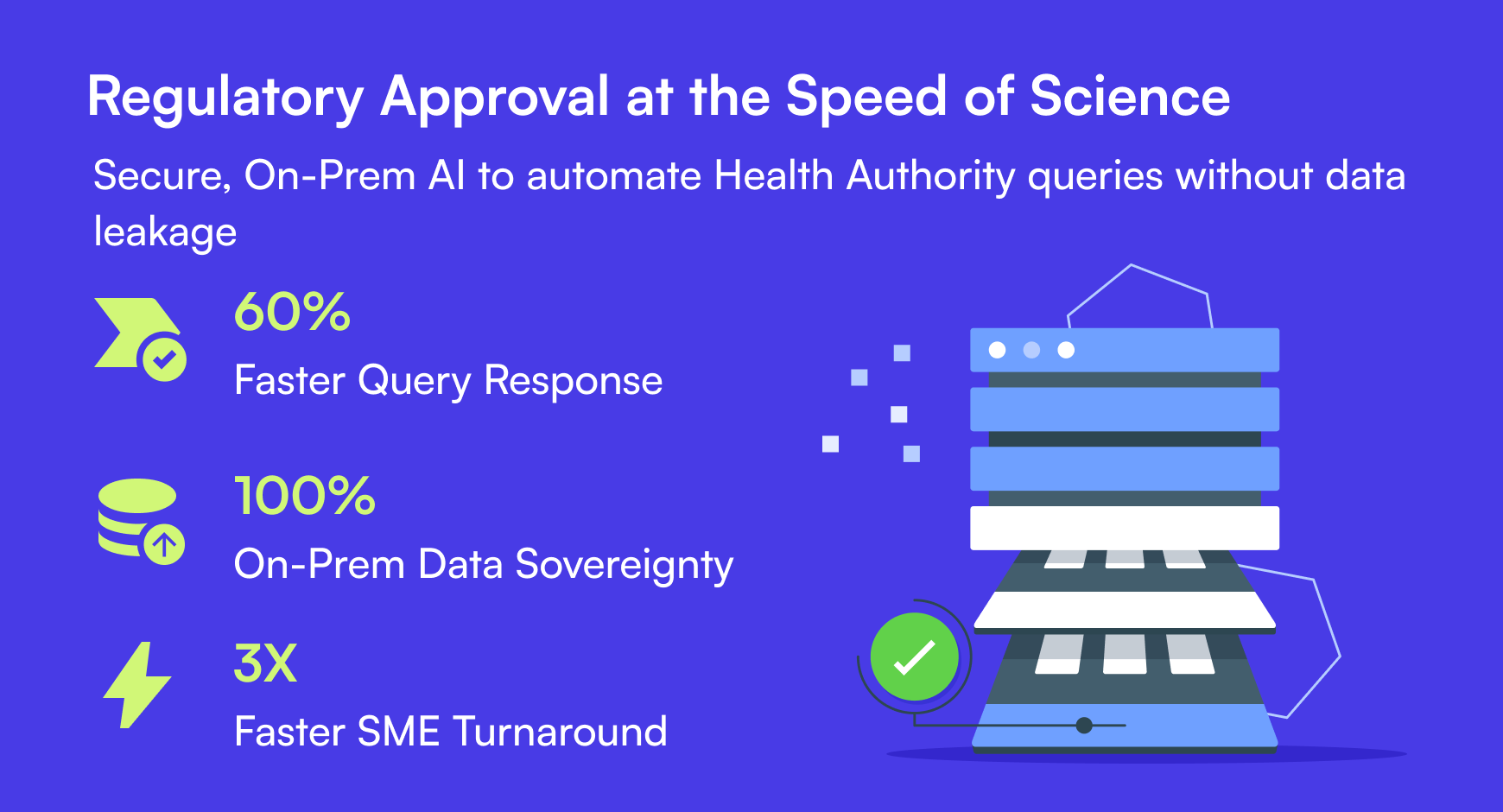
60%
100%
3X
Client Overview
For clients that are pharmaceutical companies focused on developing novel molecules. The regulatory affairs team manages complex submissions across global jurisdictions.
Every molecule approval relies on successfully answering Health Authority (HA) queries.
For these companies the goal is to bring life-saving therapies to market without delay. The business must maintain rigorous compliance standards while clearing a backlog of technical questions from regulators.
The Challenges: Data Silos, Compliance Risks, Slow Manual Search
The regulatory team faced a critical bottleneck during the approval phase. They had to address hundreds of intricate questions from Health Authorities regarding efficacy and safety. These requests were high-stakes and difficult to resolve quickly.
Critical information was fragmented across the organization. Clinical trial data lived in isolated data lakes. Historical submission logs were archived separately.
Subject Matter Experts (SMEs) kept personal records in local folders. The regulatory leads operated in email, while the data needed to answer them was buried in lab reports.
This disconnection caused several significant problems:
- SME Bandwidth Drain: Top researchers spent weeks acting as librarians. A single HA query required an SME to manually check multiple data lakes to find the right evidence. This manual hunt pulled high-value staff away from innovation to answer routine questions.
- Strict Formatting Failures: Answers often missed the mark on presentation. Health Authorities demand responses in specific, rigid formats. When engineers or scientists provided raw data, it required heavy re-formatting. Errors here caused submission rejections and delayed market entry.
- Risk of Hallucinations: The team could not tolerate ambiguity. In a regulated environment, an AI "guess" is a liability. They needed absolute certainty that every claim in a response was backed by internal data. Standard AI tools could not guarantee this accuracy.
- Data Sovereignty Constraints: Public cloud solutions were strictly forbidden. The data involved constitutes the company's most valuable intellectual property. It could not leave their secure environment. The team needed advanced AI capabilities without exposing sensitive molecule data to the internet.
Our Intelligent Solution
We secured the regulatory pipeline from within. The team deployed Thunai Brain directly onto their on-premise infrastructure. This system acts as the central intelligence for their whole operation.
Thunai Brain serves as a living, unified knowledge system. The tool connects to internal data lakes, clinical databases, and submission archives without moving the data. This integration gave the team a single, secure engine to formulate accurate responses.
This deployment allowed us to transform the submission workflow:
- Unified Secure Search: We configured Thunai Brain to ingest and understand all file types, including documents, spreadsheets, and trial reports. The system creates a Knowledge Graph that organizes content into domains. This allows SMEs to query millions of documents instantly from a single source of truth.
- Zero-Hallucination Drafting: Thunai Brain utilizes Contradiction and Relationship Resolution to ensure accuracy. The tool detects contextual conflicts across documents and supports human-in-the-loop verification. It anchors every response to specific internal evidence, eliminating the risk of unverified claims.
- Intelligent Content Generation: We eliminated manual drafting. Using the Ask Thunai intelligent chat interface, the system generates or edits documents based on enterprise data.
- Total Data Sovereignty: We ensured the data never left the building. Thunai Brain isolates sensitive data through strict compartmentalization. The entire stack runs on the client's own hardware, allowing them to use powerful AI securely over their own company data.
Conclusion
Thunai changed how the client navigates regulatory hurdles. The setup turned a manual, high-risk process into a streamlined verification engine.
The system brought scattered data lakes and compliance rules together using Thunai Brain. SMEs shifted from hunting for data to reviewing ready-made, cited drafts.
The tool also enforced mandatory formatting automatically. The client reduced query response times by 60%. The team now secures approvals for critical molecules faster, with zero data leakage.

